 No one can argue the impact battery technology has had on society; ushering in an era or portability and mobility like never before, all the while helping to spark a green revolution, and empower people with tools and ideas for a sustainable future. What’s more, emerging lithium ion technology provides significantly faster charge, longer life, and designers in the U.S. have managed to configure large lithium ion batteries for mass production and ensure they are long-lasting and safe for the auto industry.
No one can argue the impact battery technology has had on society; ushering in an era or portability and mobility like never before, all the while helping to spark a green revolution, and empower people with tools and ideas for a sustainable future. What’s more, emerging lithium ion technology provides significantly faster charge, longer life, and designers in the U.S. have managed to configure large lithium ion batteries for mass production and ensure they are long-lasting and safe for the auto industry.Risks seemingly mitigated, recent incentives and regulation will surely mean a boon for lithium technology, however, social, environmental, and political debates rage on between the Middle East, South America and the United States over control of lithium and oil resources. Furthermore, a growing global dependency on oil, coupled with the countless trillions oil producers stand to lose should lithium ion replace oil only serve to create new geopolitical tensions and promote false claims, rather than help abate existing ones.
In the course of two articles, we will discuss these issues as they pertain to emerging Lithium-Ion technologies, examine the history and engineering behind such inventions, and draw comparisons between alternative battery technologies in use today. These papers will explore the functionality of lithium batteries and investigate the pros and cons of different technologies as they relate, and aim to shed light on the associated social and environmental risks.
A Brief History of the Battery
As we have witnessed since the dawn of the industrial revolution, the complexity and pace at which technology transform society are relentless. Following the trail of events from some point in the past to a piece of modern technology is somewhat like a detective story; full of sudden twists and turns, deception, and guess work! You’ll never know where the story is heading until the last minute. Why those inventions happened is a blunderous blend of accident, genius, war, economics, religion, politics and countless other social factors involved in the business of change. The invention of the battery is no exception.
 Our story of the battery begins with the three ceramic pots uncovered during archeological excavations near Baghdad [301]. Around this time Mesopotamia (modern Iraq) was occupied by the Parthians, a nomadic tribe of skilled warriors, who had literature and kept records.[302] Yet the importance of such an unusual electrical phenomenon seems to have gone completely unrecorded within the Parthian and contemporary cultures and then to have been entirely forgotten about for almost 2000 years. Is the Baghdad battery the accidental forerunner of electricity or did the Parthians, having knowledge of electricity, invent the battery? Perhaps more conspicuously, how did this incredible piece of technology go unnoticed for 2000 years? These mysteries may never be solved, but serve as a fitting prelude to our chronicle, adding accident, genius, and perhaps deception (in the form of alien theories and misguided propaganda) to the cast.
Our story of the battery begins with the three ceramic pots uncovered during archeological excavations near Baghdad [301]. Around this time Mesopotamia (modern Iraq) was occupied by the Parthians, a nomadic tribe of skilled warriors, who had literature and kept records.[302] Yet the importance of such an unusual electrical phenomenon seems to have gone completely unrecorded within the Parthian and contemporary cultures and then to have been entirely forgotten about for almost 2000 years. Is the Baghdad battery the accidental forerunner of electricity or did the Parthians, having knowledge of electricity, invent the battery? Perhaps more conspicuously, how did this incredible piece of technology go unnoticed for 2000 years? These mysteries may never be solved, but serve as a fitting prelude to our chronicle, adding accident, genius, and perhaps deception (in the form of alien theories and misguided propaganda) to the cast.It wasn’t until 1745 that electricity was again stored in bottles, called Leyden Jars [303]; essentially a large capacitor jar filled with water, with metal foil around the outside and a nail piercing the stopper and dipping into the water. Born from necessity, Leyden jars and electrostatic generators were the scientist’s only source of electrical energy at the time and remained curiosities for amusement until Canadian Lew Urry patented the first modern alkaline battery in 1959[304], which served as the precursor to mass production by Ever Ready and Duracell starting in 1968.
Since then, developers have been focused on meeting the demands of the ever increasing consumer electronics market, which has resulted in faster, smaller, more powerful, and better quality batteries that also promise to meet the demands of the green revolution and electric vehicles in particular. In fact, the push towards renewable energy has thrust battery technology into the spotlight like never before and has resulted in a new breed of Lithium polymer batteries poised to empower a sustainable society.
| 250 BC | Baghdad battery |
| 1800 | Alessandro Volta invents the first modern electric battery |
| 1901 | Edison invents Nickel Iron battery |
| 1960 | Neumann develops Nickel Cadmium battery |
| 1970 | Union Carbide develops Lead Acid battery |
| 1991 | Sony commercialization of Lithium-Ion battery |
| 1996 | John B. Goodenough patented Lithium Iron Phosphate LiFePO4 battery |
| 1999 | Sony commercialization of Lithium Polymer battery |
| 2009 | G.Ceder & B.Kang invents “Beltway Battery” based on LiFePO4 technology |
First generation Lithium cells first appeared in the late 1980’s, however, failed to attract consumer attention due to the inherent instability of Lithium metal during charging and research shifted towards a non-metallic Lithium battery using Lithium ions which were commercialized by Sony in the early 1990s. While Lithium-ion batteries proved to be safer, they were still prone to “thermal runaways” if mishandled and had lower energy density than Lithium metal.
A non-volatile Lithium Iron Phosphate battery was developed for medical uses in 1996, but, low energy density kept it from widespread use until 2003 when the charge efficiency of Lithium Iron Phosphate batteries had improved enough to allow use in military and medical applications such as the pacemaker.
How Lithium Technology Works
A lithium-ion battery consists of a cathode layer, an anode layer, and a separator between them. When the battery is charging (figure 1), positively charged lithium ions move through a liquid electrolyte from the cathode to the anode and in the opposite direction when the battery is discharging (figure 2). The electrolyte is a lithium salt, dissolved in organic solvents. [103] Lithium batteries come in two types, primary lithium batteries (single use batteries) and secondary batteries (rechargeable batteries).
 |  |
Figure 1 – Charge Cycle | Figure 2 –Discharge Cycle |
- Slow: 14-16 hours
- Quick: 3-6 hours
- Fast: Less than 1 hour
The rate of charge is determined by how much electrical current is allowed into the battery by the charger and how fast it can force electron to flow from anode to cathode. Some batteries can handle a higher voltage in a shorter amount of time without overheating, while others need a lesser voltage applied over a longer period of time. The quicker the rate of charge, the more chance there is of overcharging. When the batteries are overcharged, the cathodes tend to release oxygen gas. The combination of oxygen, a flammable solvent, and heat can lead to a thermal runaway or cause the battery cell to explode. Faster secondary batteries use protection circuitry to balance or control electron flow to individual cells in the pack when charging. The circuit limits the peak voltage of each cell during charge and prevents the cell voltage from dropping too low on discharge. Also, chargers have built-in voltage regulators that allow cell phones, computers, and other portable device to be left plugged in for extended periods of time. In addition, in fast charge batteries the cell temperature has to be monitored to prevent temperature extremes. The maximum charge and discharge current on most packs are is limited to between 1C and 2C. (C stands for capacity)
Types of Lithium Batteries
Despite all the drawbacks, the lithium based batteries have twice the energy density of standard nickel-cadmium batteries, typically AA, AAA, or any letter based batteries. The charge characteristics are reasonably good and behave similarly to nickel-cadmium in terms of discharge. For example, a Lithium-Ion battery has a maximum individual cell voltage of 3.6volts. Most of today's mobile phones run on a Lithium-Ion single cell. A nickel-based pack would require three 1.2-volt cells connected in series.
Lithium batteries are low maintenance batteries, an advantage that most other chemistries batteries cannot claim. There is no memory and no scheduled cycling is required to prolong the battery's life. In addition, the self-discharge is less than half compared to nickel-cadmium. [106]There are several different categories of lithium batteries. Based on the type of electrolyte employed batteries can be used in verity of applications. Smaller size Lithium-Polymer batteries are used in portable devices and RC toys, Lithium-Ion have higher energy density but more riskier are used in stationary applications as backup electrical power or factory motors. Lithium Iron Phosphate batteries are used primary in medical applications and military where safety is at most importance.
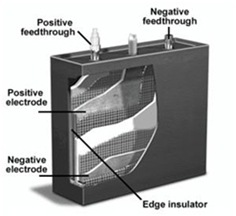 Lithium type batteries are flexible in design and can be manufactured in any cylindrical or prismatic forms. The flexibility allows batteries to be used in wide variety of portable devices, power tools, and ultra slim appliances.
Lithium type batteries are flexible in design and can be manufactured in any cylindrical or prismatic forms. The flexibility allows batteries to be used in wide variety of portable devices, power tools, and ultra slim appliances. Shown in figure 3 (left) is a cutaway view of a lithium-Ion battery. Both the positive and negative feed-through (negative and positive ends) are located on the top of the battery and become as flat plates on the inside. Additional vents can be added to help relieve oxygen build up, sensors for monitoring temperature and reinforced casing (edge insulator) to prevent explosion of a battery if overcharged. [107]
Lithium Ion Batteries (Li-Ion)
Rechargeable Lithium Ion (Li-Ion) cells have a negative electrode (anode) made from lithium compounds. Lithium is a highly reactive material and is much lighter than the hydrogen-absorbing metal alloy of the Nickel-metal hydride battery (NiMH) negative electrode. This leads to higher gravimetric energy densities for the Li-Ion cell. [109]
Advantages | Disadvantages |
|
|
Lithium Polymer (Li-Po)
Lithium Polymer Ion batteries provide the performance of the Li-ion in a thin or moldable package. They do not use a volatile liquid electrolyte and can sustain significant abuse without explosion or fire. The lithium polymer uses a polymer gel electrolyte to replace the traditional liquid electrolyte. Lithium-polymer finds its market niche in wafer-thin geometries, such as batteries for credit cards and other such applications. [110]
Advantages | Disadvantages |
|
|
Lithium Iron Phosphate batteries (LiFePO4) were developed in 1996 by Dr. John B. Goodenough and his research team at University of Texas. His invention was patented and manufacturing permission was given to Phostech Lithium Inc. and Hydro Quebec. [111]
This type of battery is based on the existing Lithium Ion chemistry however, rather than using lithium cobalt oxide (LiCoO2) as a cathode, LiFePO4 uses Iron (Fe). Unlike cobalt, iron is non-volatile and does not produce oxygen. This means that even under severe operating conditions such as overcharging, deep discharging, or fast charging (which usually causes thermal runaway), LiFePO4 batteries will not burn or catch on fire. Because of this property, this type of battery can be installed in confined places or ones without ventilation. Furthermore, due to their slim cylindrical nature, custom batteries of virtually any shape can be made that will fit into tight space.
Another great advantage of LiFePO4 batteries is their extremely long life. If a battery left sitting on the shelf without use, it can last up to 20 years. Also LiFePO4 battery can typically be charged in excess of 2000 times and there are LiFePO4 cells which are currently under test at the US Department of Energy Laboratories in New Mexico which have recently passed 7000 cycles and are still working. [112]
Another advantage of these batteries is their rapid charge capability. LiFePO4 batteries can be re-charged extremely quickly. This rapid charge capability comes by necessity since these batteries have been developed for use in the electric cars in the near future. High end LiFePO4 batteries can be charged at 30C rate. This safety combined with their light weight has found wide use for these batteries for military and medical applications and now for the emerging electric vehicle market. However one trade off to safety is LiFePO4 does not have as high energy density as Lithium-Ion or Lithium-Polymer battery.
An overview of the benefits of Lithium Iron Phosphate batteries: Advantages | Disadvantages |
|
|
LiFePO4 batteries have only been commercially available on the market since 2008, first manufactured by A123Systems [113] for electrical bicycles and smaller electrical cars. As batteries became more popular, today more than a dozen battery companies produce them. Fist laptop size battery was used in XO children laptop in November of 2007, and cell phone size batteries were expected to be available in later 2010. [114]
Next Generation Lithium Battery
State of the art rechargeable LiFePO4, although having high energy densities, still have relatively slow power rates. Even at 30C discharge rates compared to Lithium-Ion batteries, improvement can still be made. A recent publication in March 2009 Nature magazine by Byoungwoo Kang and Gerbrand Ceder of Massachusetts Institute of Technology (MIT) claims a breakthrough with a small prototype battery made on existent LiFePO4 technology that can be charged fully in 10 to 20 seconds, compared to 6 minutes for the cell made in the standard way. [116]
The idea for the research came when Ceder questioned why electrical vehicles using Lithium batteries can drive continuously at 55mph but have very low power rates – how fast the car can accelerate. [117]
Traditionally scientists have thought that lithium ions responsible, along with electrons, for carrying charge across the battery simply move too slowly through the material. About five years ago Ceder a graduate student in material science and engineering made a surprising discovery. Computer calculations of lithium ions transfer in LiFePO4 showed that ions should move extremely quickly. “If transport of the lithium ions was so fast, something else had to be the problem” Ceder said. [118] A deeper looking into calculations revealed that lithium ions can move very quickly into the material but only through tunnels accessed from surface. “If lithium ion is at surface directly in front of the tunnel entrance, there is no problem: it processes efficiently into the tunnel, however if the ion is not directly in front, it is prevented from reaching the tunnel entrance because it cannot move to access the entrance.” [118]
A way around it was modifying Lithium Phosphate material in electrode to allow ions and electrons move in and out much quickly. By including extra Lithium and Phosphorus helped created a layer of Lithium Diphosphate (Li4O7P2) on the surface. Only 5nm wide, Lithium Diphosphate has high lithium-ion conductivity, and ions and electrons that come in contact with it quickly shuttled to faces that can pull them in, allowing for very fast tunnel access.
The technology has been nicknamed the “beltway battery”, after the orbital motorway in Washington DC, because it uses a bypass system to let lithium ions that carry charge to enter and leave the battery more quickly. [121] Figures 6 and 7.
 | 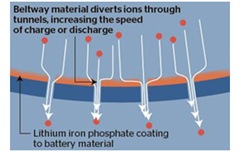 |
| Figure 6 Lithium Ion flow in a traditional battery [121] | Figure 7 Ion flow in the Beltway Battery [121] |
To better illustrate the capabilities of the LiFePO4 batteries with Lithium Di-phosphate coating the fallowing example might be helpful. A high end laptop Lithium Polymer battery has fallowing specifications; Capacity of 2100mAh at 11.1V thus it can provide 23Watts-Hour of Power. Also it has a 20C continues discharge rate, and 1C charge rating. Capacity denoted as C and signifies a charge or discharge rate equal to the capacity of a battery divided by 1 hour. For example 1C for a 2100 mAh battery would be 2100 mA or 2.1 Amps for one hour, if discharges at twice the capacity (2C) the battery will give more current (4.2Amps) but will last less (30 minutes compared to original 60) The durations are summarized in the table below.
| Li-Polymer | Current | Duration |
| 1 C charge/discharge | 2.1 Amps | 1 hour |
| 2C discharge | 4.2 Amps | 20 min |
| 20C discharge | 42 Amps | 3 min |
As mentioned, the problem with standard Lithium-Polymer batteries are low discharge and even lower charge rates. However LiFePO4 batteries with Lithium Di-phosphate coating can have a charge and discharge rate of 100 times its capacity. The same Lithium Polymer battery from above, can be charged or discharged at 100C, thus it can charged at staggering 210 Amps in only 36 seconds. By comparison, 210 Amps at 11.1V equals to 2.3kWatts of power. This is equivalent to 23 100Volt light bulbs turned on for 36 seconds.
The Impacts of Society on Battery Technology
In Part 2 of this series we will explore the impacts of society on battery technology and look at how emerging Lithium Iron Phosphate technology will change how we use battery powered devices.


Wow! real scientific information. What a refrehing change from the glitz of prematurely released news from soon to be forgotten fundseeking companies. I'm new here and I think I'm gonna love it, love it madly
ReplyDelete