This proposal aims to provide the design, implementation and operation of an environmentally sound anti-icing process that safely and effectively fulfills Montreal’s road management needs. This strategy would significantly reduce the amounts of chlorinated salts entering the environment, reduce operational and maintenance costs, help mitigate the current condition ailing Montreal’s infrastructure, and improve road safety, in addition to provide a better understanding on the fate of alternative designs on the environment. Deliverables for the project include but are not limited to:
- Design specifications for alternative brine solutions.
- Design specifications for equipment including storage and Real Weather Information Systems (RWIS) test platform.
- Prepare Anti-icing Standard Operation Manual and training.
- Prepare Life Cycle Estimates of alternatives.
- Provide ecotoxicology estimates on priority species.
- Start up and operational budgets. Timeline for Implementation.
The Problem with Salt
“Based on the available data, it is considered that road salts that contain inorganic chloride salts with or without ferrocyanide salts are entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Therefore, it is concluded that road salts that contain inorganic chloride salts with or without ferrocyanide salts are ‘toxic’ as defined in Section 64 of the Canadian Environmental Protection Act, 1999 (CEPA 1999)”. [100]
Loading
Road salts came into common use in the 1940’s as a melting agent for removing ice and snow. Driven by growing urbanization and increasing density of road networks, as well as changes in services levels requiring more “bare pavement” policies on roadways [101], total road salts usage and application rates have been on the rise in Canada since the 1970’s.

Figure 1 - Historical salt use by provincial agencies (Salt Institute, 1964-1983), Morin and Perchanok, 2000 [106]
Today, an average of 5 million tones of road salts are used each year as deicing agents on roadways in Canada [103], yet there are no federal regulations which directly govern levels of salt use, or salt concentrations in various environmental media. In fact, the only relevant national environmental standards, which relate indirectly to road salt in the environment, are the Guidelines for Canadian Drinking Water Quality, March 2001. The aesthetic objective having a maximum of 200 mg/L sodium and 250 mg/L chloride.[100] Using a conservative estimate of road salts usage in Montreal; approximately 70,000 tons of salt is applied each year which is fated for the St. Lawrence with a flow rate of 9000 m3/s around the Island, resulting in a steady flow concentration of less than 0.25 ug/L. Consequently, much of the salt fated for the St. Lawrence basin does not appear to be of immediate concern.
However, water bodies most impacted by road salts are small ponds and watercourses draining large urbanized areas, as well as streams, wetlands or lakes draining major roadways. Waters from roadways, patrol yards or snow dumps can be diluted to various degrees when entering the environment, resulting in chloride concentrations as high as 2800 mg/L in groundwater adjacent to storage yards, 4000 mg/L in ponds and wetlands, 4300 mg/L in watercourses, 2000–5000 mg/L in urban impoundment lakes and 150–300 mg/L in rural lakes.[102] While highest concentrations are usually associated with winter or spring thaws, elevated concentrations can still be measured in the summer, as a result of the travel time of the ions to surface waters and the reduced water flows.
In water, natural background concentrations of chloride are generally less than a few milligrams per liter, however, concentrations of chloride over 18 000 mg/L were observed in runoff from roadways and chloride concentrations up to 82 000 mg/L were also observed in runoff from uncovered blended abrasive/salt piles in patrol yards. Chloride concentrations in snow cleared from city streets can be quite variable. For example, the average chloride concentrations in snow cleared from streets in Montréal were 3000 mg/L for secondary streets and 5000 mg/L for primary streets in the winter of 1997-98. [104]
Pathways
Ultimately, all road salts enter the environment as a result of: storage at patrol yards, roadway application, and disposal of waste snow. Releases are therefore associated with both point sources (storage and snow disposal) and line sources (roadway application). Delisle and Dériger [105] reviewed the physicochemical and ecotoxicological characteristics of roadside snow and the different sources related to snow removal from roads and sidewalks.
Disposal of Waste Snow
Clearing of snow generally involves plowing snow to the side of the roadway. In cities, snow clearing can begin as snowfall reaches 2.5 cm, with the snow being pushed to the sides of streets and onto sidewalks. However, when there is considerable accumulation (more than 10 cm) or when necessary, snow is cleared and transported to various disposal sites (City of Montréal, 1998). [107] The physicochemical and ecotoxicological characteristics in relation to various snow disposal methods have been reviewed by Delisle and Dériger and can be grouped into three categories, as described below.
Methods for clearing snow from the roadway without transporting it to snow disposal sites typically involve plowing snow to the side of the road or blowing it onto land adjacent to the roadway. These methods are generally not effective in areas with a high land occupancy factor.
Removal and disposal of snow may be required when the accumulation of snow on or along roadways may hamper traffic or safety. As such, the quantity of snow to be removed and disposed of depends on the volume of snow and the extent of urban development. Given the large amount of snowfall in Montréal and the size and density of the city, about 11.258 million cubic meters of snow were brought to snow disposal sites during the winter of 1997–98 (Figure 2).

Figure 2 - Total volume of waste snow and salt used in certain Canadian cities [105]
Roadway snow can be transported to snow disposal sites where it melts and the meltwater is treated. This typically involves dumping snow at surface sites or in quarries where runoff is channeled to treatment facilities. Generally, snow disposal sites are located on impermeable or slightly permeable ground or must be equipped with a geotextile membrane. Some sites are also equipped with sedimentation facilities or are designed to direct the meltwater towards a wastewater treatment system . These sites should not be located next to watercourses that could be affected by runoff.
Pinard et al. (1989) characterized chloride concentrations in runoff from snow disposal sites. This study indicated that only 2% of the salt spread on city streets was present in meltwater from snow disposal sites, with most of the salt likely released to the environment from the roadway or roadside.[109] This concurs with Delisle and Leduc (1987), who indicated that chloride concentrations in roadside snow initially increase then decrease with increasing time. [110]
While the percentage of salt present in snow transferred to snow depots may be low (e.g., 2%), the concentration of chloride in runoff is still elevated. A study by Péloquin (1993) indicated that the average chloride concentration in meltwater from a snow disposal site was 414 mg/L. [111] Pinard et al. (1989) monitored chloride concentrations in runoff from a Québec City snow disposal site from April 18 to the end of June 1988. Concentrations in runoff ranged from approximately 100 to 1100 mg/L. [109] Concentrations were highest in the early sampling and gradually decreased throughout the spring.
Methods involving release to surface water without water treatment. This method of disposing of snow involves dumping snow directly into a waterway or onto its banks. Snow can also be dumped down sewer chutes that are not linked to treatment plants. This results in the release of waste snow and any contaminants to surface water directly (dumping into rivers or into the ocean), with some removal of large debris (dumping onto banks) or with possible dilution by stormwater (dumping into sewer chutes not linked to treatment plants).
Roadway Application
Salt is typically applied in either a proactive approach prior to ice or snow formation or as a reactive approach after the precipitation has bonded to the pavement. Anti-icing is the proactive procedure that involves applying material prior to snow or ice formation to prevent ice from forming on the pavement, resulting in less material usage and fewer snowplow trips than a deicing strategy. Deicing is the reactive snow and ice control strategy that involves applying material on top of a layer of snow or ice that is already frozen or bonded to the surface of the pavement. Once bonded to the pavement, ice cannot be removed by plowing, and salt must then be applied to break the bond by lowering the freeze point of water.
 A spreader with a spinner is the most common way of applying deicers. A spinning circular plate throws the de-icer out in a semi-circle. Alternatively, a chute can distribute deicer in a windrow on the road, usually on the centerline for best performance. Spreaders can be equipped with automatic or ground-oriented controls, which automatically regulate application rates as truck speeds fluctuate and have proven effective in reducing waste chemicals. Different materials will spread at different rates at the same spreader control setting, so calibration is essential for controlling application rates and must be verified on a regular basis.
A spreader with a spinner is the most common way of applying deicers. A spinning circular plate throws the de-icer out in a semi-circle. Alternatively, a chute can distribute deicer in a windrow on the road, usually on the centerline for best performance. Spreaders can be equipped with automatic or ground-oriented controls, which automatically regulate application rates as truck speeds fluctuate and have proven effective in reducing waste chemicals. Different materials will spread at different rates at the same spreader control setting, so calibration is essential for controlling application rates and must be verified on a regular basis.Pre-wetting techniques can be used to moisten the salt with brine solution with either on-board pre-wetting systems or by pre-treating stockpiles, to keep the salt on the roadway during applications. Pre-wetted salt clings to road, which minimizes non-target applications and uses less salt. Wet salt also works at lower temperatures (generally below -6ºC) by accelerating the production of brine.
Sand and other abrasives are often necessary when temperatures fall below the eutectic point of the saline solution to improve vehicle traction on snow and ice covered roads. Sand is the most common abrasive, but slag, cinders, and bottom ash from power plants are also used. Abrasives used for winter road maintenance can have negative impact on infrastructure, clogging storm water inlets and sewers. Cleanup may be necessary in urban areas, on bridge decks, and in ditches. The materials may wash downstream and end up in streams and lakes. Abrasives must be treated with salt to keep them unfrozen and usable whereas salt-treated abrasives can accelerate vehicle corrosion. Recent concern has been raised in areas with air pollution with particles less than 10 microns in size (pm 10) having been documented from winter abrasive use. [108] As a result, cleaner abrasives and quicker cleanup after the storm are being required in areas with severe air pollution problems.
Patrol Yards
Patrol yards (also referred to as storage yards or maintenance yards) are used to store road maintenance materials before their application to roadways. Patrol yards can be located in a variety of settings, and patrol yard design and standards vary substantially across Canada. Accordingly, the degree of protection against weathering varies considerably. Covered facilities used to store road salts can include domes/igloos, sheds and leantos. Doors or walls may or may not be present, and storage under an overpass can be considered as covered by some agencies. Salt may be stored on asphalt or concrete pads or outside on a thick plastic tarp and covered by another tarp.
A New Brunswick study (NB DOE and DOT, 1978) monitored the quantity and quality of leachate from a 2000-tonne abrasive pile with a 2.5% salt content. During the first year, 420 m3 of leachate passed through the monitoring system. Sodium and chloride concentrations in the leachate are depicted in Figure 4 below.

Figure 4 - Average daily concentration of leachate from salt-treated sand pile, 1975–76
Estimates of releases to the environment were made by Snodgrass and Morin (2000). Figure 5 below provides three estimates based on the following scenarios: A patrol yard with best management practices where salt and mixed abrasive piles are stored indoors; a patrol yard where salt piles are stored indoors and abrasive piles are stored outdoors; and a patrol yard here neither abrasive piles nor salt piles are stored indoors. Of the three scenarios, options 1 and 2 are probably most representative of patrol yards. [112] The last column of figure 5 estimates the percentage of total road salt use that may be lost at patrol yards. Depending on the facility and salt management options, 0.2–20% of total salt use can be lost at patrol yards.
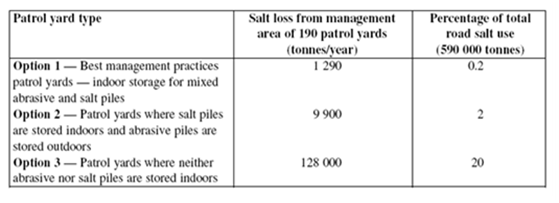
Figure 5 - Estimate of magnitude of salt loss at patrol yards
Ville-Marie Case Study
Given the population density of Montreal (423 pers./km2), clearing snow from the roadway without transporting it to snow disposal sites is not feasible. Furthermore, the direct dumping of snow to fresh surface water is restricted and is not permitted in Quebec as of 2002 [104]. In the past, municipalities such as Montréal blew snow onto private property and occasionally used snow melters. Blowing onto private land was virtually abandoned by Montréal because of social and political pressure, and snow melters proved to be too expensive to operate because of fuel costs. Furthermore, in response to exceptional snow fall in 2002-03, Montreal instituted a strategic plan to improve snow removal operations in 2008-09. [113]
Disposal Methods
The 2008-09 strategic plan for snow removal operations is a coordinate effort among the 14 boroughs on the island which aims to; review the training plan for foremen and adapt it to snow removal operations, improve mechanic availability all winter long, prepare two temporary sites ahead of time, provide work on the Saint-Michel quarry and sewer chutes, and improve site performance.

Figure 6 - Ville-Marie Disposal Sites
As a result, Montreal currently uses 30 disposal sites to treat 13.5 million m3/year of snow collected: 13 snow dumps (49%), 16 sewer chutes (32%), and 1 dedicated quarry (19%). Figure 6 shows the relative location of chutes and dump sites available to Ville-Marie operations.
Storage & Application Methods
Ville-Marie currently stockpiles salt, sand, and gravel under the Ville-Marie Expressway located at its depot. Figure 7 shows stockpiles of gravel (left) and salt (right) left over from 2008 operations. Concrete walls and pads separate the materials and storage under the overpass is considered as covered. Each year over 120,000 tones of road salt and 13,000 tons of sand or gravel are dumped on Montreal’s streets which are fated to wind up in the environment or have negative impacts on infrastructure.

Figure 7 - Ville-Marie salt and gravel stockpiles
Materials are loaded by tractor into trucks equipped with a hopper and spreader for transport and application. Shown in the various figures below are pieces of equipment used by Ville-Marie for road operations. Montreal primarily uses 3 types of spreaders (figure 8-10) to distribute salt/sand/gravel mixes as outline in their operating manual; however, Ville-Marie does have the technology and equipment able to apply liquid agents as seen in figures 11-13. When temperature fall below -15º, abrasives (salt/sand) are mixed to produce friction on road surfaces where NaCl alone is ineffective. Show in figure 14 is a cave-in located within the Ville-Marie borough and is typical of the day-to-day operational difficulties being encountered with the city’s ailing infrastructure and increased use of abrasives. Particles are forced through the system by gravitational flow until an obstacle is encountered where they stock pile, resulting in a concomitant head loss in addition to abiding erosion and other adverse conditions.

Figure 14 - Ville-Marie pipe burst and cave-in
Proposed Solutions
While it is true that current salts usage has taken its toll on Montreal’s ailing infrastructure, it is easy to foresee that a properly designed anti-icing process will alleviate the need for abrasives all together and therefore work towards mitigating the growing infrastructure problems. Moreover, due to concerns about the large quantities of chlorides being released to the environment, road salts underwent a comprehensive five-year scientific assessment under the Canadian Environmental Protection Act, 1999 beginning in 1995. The study was in reaction to a U.S. Salt Institute study which was deemed unreliable enough for Canadian climates. The road salts assessment covered the chloride salts; sodium chloride (NaCl), calcium chloride (CaCl2 ), magnesium chloride (MgCl2) and potassium chloride (KCl) , as well as brines used in road de-icing/anti-icing. [114]
This study produced two pieces of documentation; Salt Management Guide and Syntheses of Best Practices, which provides guidance available to users of road salts in Canada. Summarized below are the keys issues relating to the reduction of salt impacts in Montreal.
- Patrol yards: Measures and practices should therefore be considered to ensure storage of salts and abrasives to reduce losses through weathering, to reduce losses during transfers and to minimize releases of stormwater and equipment washwater.
- Roadway application: The selection of alternative products or of appropriate practices or technology to reduce salt use should be considered while ensuring maintenance of roadway safety.
- Snow disposal: Measures to minimize percolation of salty snowmelt waters into soil and groundwater at snow disposal sites should be considered. Measures should also be considered to ensure sufficient dilution before release.
- Sodium chloride pre-wetted with calcium chloride brine has been recommended for reducing total salt applications. [115]
Pre-Wetting
There are tremendous benefits to the pre-wetting technique, most notably the quick response in deicing, reducing salt use, and controlling non-target applications including the bouncing of dry salt on roads during application, wind, or traffic, however, there are other factors to consider when using this method. These include additional equipment for liquid storage and secondary containment, truck mounted on-board pre-wetting equipment and dependable maintenance procedures to prevent equipment corrosion, clogging, and seizing. Figure 15 shows a list of common substances along with critical parameters. While any liquid de-icing chemical can be used to pre-wet, liquid calcium chloride is used widely. Applications of 40 L/m3 are recommended.
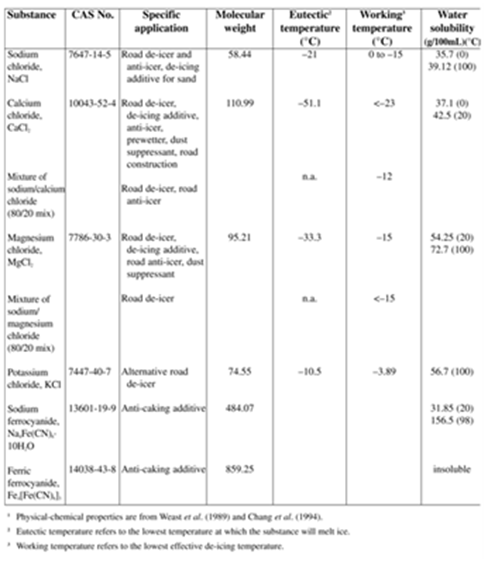
Figure 14 – Physical/Chemical properties of salts
Using salt brine to pre-wet is becoming more common because of its lower cost. Some agencies are producing their own salt brine solution from existing stock piles or turning to alternatives such as liquid calcium chloride and magnesium chloride. Additionally, new uses for by-products are finding their way into the mix. Several patents in the U.S. have designed new products which can be applied to existing stock piles. By-product from local waste streams are blended with magnesium chloride or calcium chloride to produce a liquid which can be applied directly to salt piles or during application though spray nozzles.
While some agencies spray the salt as it is loaded into the truck, the application is more uniform if truck-mounted equipment is used to spray the salt as it leaves the spreader. This also eliminates the problem of handling pre-wetted salt that is not immediately used. Traditional snow and ice control practice like Montreal’s is to wait until an inch or more of snow accumulates before beginning to plow and treat the roads with chemicals and abrasives. While this procedure is straight-forward, it frequently leads to a compacted snow layer (pack) that is tightly bonded to the pavement surface. A subsequent "deicing" of the pavement is then necessary, usually requiring a large quantity of chemical to work its way through the pack to reach the snow/pavement interface and destroy or weaken the bond. Because this operation is reactionary, it requires less judgment than anti-icing. Yet as a result of its inherent delay, it often provides less safety at higher cost. Nonetheless, the reactive technique of deicing will remain important for snow and ice control, as there will always be conditions that preclude preventive operations such as higher priority routes during storm events or heavy precipitation.
ANTI-ICING
Application of deicing agents onto roadways at the start of or prior to winter precipitation inhibits the development of a bond between the snow or ice and the pavement surface. Moreover, less concentrated and periodic reapplications of the chemical during precipitation will continue this effect. In fact additives such as beet derivates and distillers grains can decrease the bounce and increase stickiness, which promote build on road surfaces.
Preventive operations are the core of an anti-icing program that provides a maintenance manager with two major capabilities: maintaining roads in the best conditions possible during a winter storm, and to do so effectively. Anti-icing has the potential to provide the benefit of increased traffic safety at the lowest cost. However, to achieve this benefit the maintenance manager must adopt a systematic approach to snow and ice control and must ensure that the performance of the operations is consistent with the objective of preventing the formation or development of bonded snow and ice. Such an approach requires use of considerable judgment in making decisions, requires that available information sources be utilized methodically, and requires that the operations be anticipatory or prompt in nature.
As such, anti-icing is better suited to routes with a higher level of service. This is because the vigilance and timeliness of successful anti-icing operations are most compatible with service levels requiring earlier and higher frequency winter maintenance operations. It is also because the preventive nature of anti-icing can support higher service level objectives such as maintaining bare pavement throughout a storm or returning to bare pavement as soon as possible following pack formation. In fact, because of the demanding requirements of higher service levels, many maintenance forces in Montreal have been instinctively implementing elements of anti-icing practices for years and boroughs around the island have instituted successful anti-icing programs. [116]
The Insurance Corporation of British Columbia (ICBC) has recently produced a guideline entitled “Proactive Guide to Snow and Ice Control: A Guide for Highway Winter Maintenance Personnel,” which provides a comprehensive description of anti-icing application techniques which provide maximum road safety at minimum cost.[e] At the end of a two year study conducted by the ICBC, it was found that there was a reduction in the number of collisions by up to 73% on pre-treated sections, the total accident claims for the entire jurisdiction under study were reduced by 6% on snow days, resulting in an estimated savings of up to $600,000 per winter to ICBC.[117]
In accordance with these guidelines, an effective winter maintenance program consists of several elements with varying degrees of importance depending on the size of the operational jurisdiction it covers and the complexity of its road network. One element, level of service (LOS), is important for all jurisdictions and must be considered along with the climatic conditions in the design of any snow and ice control program. Figure 16 depicts the components of an anti-icing program in the context of a winter maintenance program and the LOS assignments.
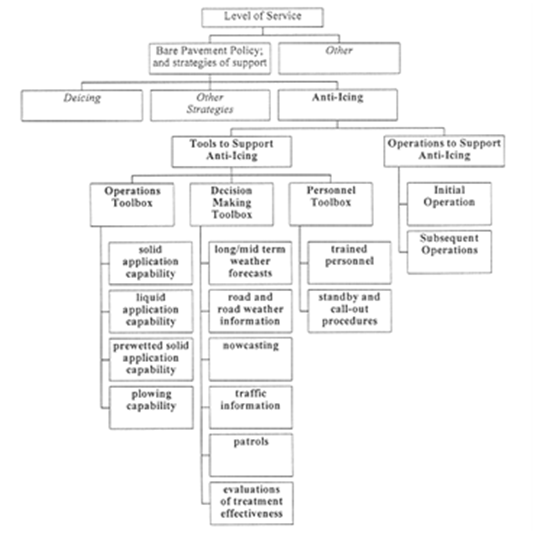
Figure 15 - LOS assignments for a winter maintenance operation
In addition to the service level, maintenance effort will vary with climatic conditions. Pavement temperature directly influences the formation, development, and breaking of a bond between fallen or compacted precipitation and the road surface as well as the effectiveness of chemical treatments and is therefore an important factor to monitor. It is also important when high humidity levels are accompanied by low dew point temperatures. Under these conditions there will be a greater potential for formation of frost and black ice. Unless some external source of heat is provided, the pavement temperature will generally track air temperature with a time delay. For road sections without obstructions to a clear sky view, higher solar radiation and exposure to the clear night sky will affect the road surface temperature to a greater extent than sections influenced by air contact only. Other important climatic factors are type and rate of precipitation.
Estimations of road salt use based on winter air temperature by the Finnish Meteorological Institute [119] found warmer than normal weather in November and March and colder than normal weather in December, January and February reduces the need for salting. Warm mid-winter months mean slippery conditions and, consequently, an abundant use of salt. The temperature explained about 60% of the annual variation of salt use and thus gave surprisingly good estimates for the required LOS. Air temperature is measured reliably at almost all meteorological stations and the use of air temperature for the estimation of road maintenance conditions is a tempting alternative compared with the use of more complicated indices calculated using meteorological observations available at only a small number of stations also referred to as the Road Weather Information System (RWIS).
RWIS is a system of sensors connected together to provide real time, accurate and site specific pavement surface conditions and weather data. Individual RWIS sites are sometimes referred to as remote processing units (RPU’s), consisting of several atmospheric sensors mounted to a tower, sensors embedded within and below the pavement surface, with all connected to a data processing unit and communications equipment. Initial research indicates Montreal currently uses RWIS technology, although it is not clear to what extent this data is applicable to Ville-Marie’s condition. Nonetheless, a study conducted in Northern Europe has demonstrated a correlation between air temperature and anti-icing application rates with a good degree of accuracy at predicting anti-icing measures on a day-to-day basis.
It is proposed that in the absence of RWIS data a test station be designed using off the shelf components for on-site monitoring that would approximate the high and low condition of an average Ville-Marie precipitation/storm event . Inclined and horizontal pavements with partial covers would be designed with sensors embedded in the pavement at critical locations. Before or during a precipitation event, data could be collected in order to calculate the level of service required.
ECOTOXICITY ESTIMATES
While numerous studies have been conducted regarding the effects of NaCl on a wide variety of species, more often, they are focused on crop producing plants. Consequently, little is known about the effects NaCl, MgCl, CaCl, or other additives on native species that once were in abundance along the St. Lawrence basin, but are currently on Environment Canada’s priority list as endangered and vital to the survival of the St. Lawrence basin.[118] The goal of this section would be to suggest a species of native plant most resilient to the proposed salt loadings as a remediation process for river embankments along the St. Lawrence in addition to provide ED50 (Effective Dose) and LD50 (Lethal Dose) estimates of the proposed alternatives on those plants. Following is a brief study plan outlining the stages of completion:
- Select three plants from Environment Canada's priority list for the St. Lawrence.
- Sow seedlings of these plants hydroponically in rock wool and varying concentrations of alternative chemicals to determine by visual inspection; a) the ED50 and LD50 of seedlings, b) test the hypothesis that seedlings sown in elevated concentrations of salts are later more resilient and grow faster under similar conditions.
- Once the first leaf has emerged, transplant into a hybrid hydroponic system and continue to grow the plants which survived stage 1 in concentrations of different alternatives to determine by visual inspection; the ED50 and LD50 of the respective species. In addition, test the hypothesis that some levels of these components are beneficial to plant growth.
The study would take about 5 weeks to grow seedlings to first leaf and complete stage 1 using existing Concordia University greenhouse and hydroponic equipment left over from a previous PhD thesis. Stage 2 would be continued until for another 2 months or sufficient points are collected along the growth stage to determine the LD50 and ED50. Time permitting; the fruiting stage will be examined to determine if there are any adverse/beneficial effects of the alternatives by assay of root stocks and fruiting matter although evidence suggests there is no benefit.
In 1974, G.P. Lumis, Department of Horticulture Science, and G. Hofstra and R. Hall, Department of Environmental Biology, University of Guelph, published results of their research on Salt Damage to Roadside Plants which will serve as a guide towards characterizing symptoms specific to each species. The study will be conducted by experienced team member(s) in a controlled environment and in accordance with Good Laboratory Practices (GLP) and prepared protocols. Concordia lab personnel have been given the mandate to work towards our project goals and will perform most of the laboratory testing as required in accordance with test protocols (Appendix B). All aspects of the study will be monitored by CCTV and data will be recorded at regular intervals using a laptop and web-cam connected to the internet. In addition to visual inspection of the plant systems, regular measurements of leaf formation, stock lengths, bulk weights, and various other growth variables and disease characteristics will be recorded to determine the ED50 (Effective Dose) and LD50 (Lethal Dose) for particular species.
PILOT STUDY
Having accumulated sufficient background knowledge of the process, a pilot study involving trained Ville-Marie personnel and retrofitted equipment (See figure 8 - 10) would be conducted in accordance with prepared operating procedures using applicable anti-icing techniques to determine what measures specific to their needs should be addressed in the final proposal. Over a period of time, weather forecasts will be monitored; LOS will be calculated by team members and conveyed to management and maintenance crews who then will apply the LOS required. Team members will monitor each step of the process and perform LOS estimates after each application.
LIFE CYCLE ASSESSMENT
The goal of any good design is to support a continuing effort to improve and maintain environmental quality by reducing energy and materials consumption and by minimizing the impacts of pollution generated by the production, use and disposal of goods and services available to Canadians.
Based on a review of currently available life cycle information, mitigation of salt requirements will produce an environmental benefit through the reduction of harmful chlorinated air emissions and water effluents adverse effects on species. Therefore, the goal of this endeavor is to provide future policy makers with a more thorough life cycle analysis specific to Montreal’s condition and the alternative available.
Toward these endeavors, Life Cycle Assessment (LCA) is increasingly being used in waste sectors in Denmark which includes input of energy and resources as well as output of waste and emissions to air, water and soil. More importantly, acidification and ecotoxicity are examples of environmental impacts that are assessed in the LCA. [121] These and other research material will serve as guidance documents which form the basis for this assessment.
References
[100] Environment Canada, “CEPA Environmental Registry”, Canada Gazette Part I, Vol. 135 No. 48, Dec. 2001[101] Terry, Robert C, “Road salt, drinking water, and safety; improving public policy and practice”, Cambridge, Mass., Ballinger Pub. Co. ,1974
[102] Federal-Provincial Subcommittee on Drinking Water (Canada) , Guidelines for Canadian drinking water quality / prepared by the Federal-Provincial Subcommittee on Drinking Water of the Federal-Provincial Advisory Committee on Environmental and Occupational Health, 6th ed , Ottawa : Health and Welfare Canada, 1996
[103] Environment Canada, “Risk Management Strategy for Road Salts”, 2006, http://www.ec.gc.ca/nopp/roadsalt/reports/en/rms.cfm
[104] Priority substances list assessment report : inorganic chloramines Publisher [Ottawa] : Environment Canada : Health Canada, c2001
[105] Delisle, C.E. and L. Dériger. 2000. “Caracterisation et élimination des neiges usées : impacts sur l’environnement”, Report submitted to the Environment Canada CEPA Priority Substances List Environmental Resource Group on Road Salts. Commercial Chemicals Evaluation Branch, Environment Canada, Hull, Quebec.
[106] Morin, D. and M. Perchanok. 2000. Road salt loadings in Canada. Supporting document for the road salts PSL assessment. Report submitted to the Environment Canada CEPA Priority Substances List Environmental Resource Group on Road Salts, May 2000. Commercial Chemicals Evaluation Branch,Environment Canada, Hull, Quebec. 85 pp.
[107] Ville de Montréal , “Measures to improve snow removal operations“, 2009, http://ville.montreal.qc.ca/portal/page?_pageid=5637,30739593&_dad=portal&_schema=PORTAL
[108] Wisconsin Transportation Center, “Wisconsin Transportation Bulletin No. 6: Using Salt and Sand for Winter Road Maintenance", 1996
[109] Pinard, D., J.B. Sérodes and P.A. Côté. 1989. “Charactérisation des eaux de fonte d’un dépot à neiges usées.” Sci. Tech. Eau 22(3): 211-215.
[110] Delisle, C.E. and A. Leduc. 1987. Évolution dans le temps et dans l’espace de la qualité de la neige usée et de l’eau de ruissellement de pluie du territoire de la Ville de Montréal. Centre de développement technologique de l’École Polytechnique de Montréal. Final report. 155 pp. + annexes.
[111] Péloquin, Y. 1993. Qualité des eaux de fonte provenant d’un site de surface pour l’élimination des neiges usées à la Ville de Laval. Université du Québec à Montréal, Montréal, Québec. 91 pp.
[112] Snodgrass, W.J. and D. Morin.,”Patrol (maintenance/works) yards. Supporting document for the road salts PSL assessment”, July 2000, Commercial Chemicals Evaluation Branch, Environment Canada, Hull, Quebec.
[113] Ville de Montréal , “Measures to improve snow removal operations“, 2009, http://ville.montreal.qc.ca/portal/page?_pageid=5637,30739593&_dad=portal&_schema=PORTAL
[114] Government of Canada,” Notice with respect to the Code of Practice for the Environmental Management of Road Salts”, 2009 http://www.gazette.gc.ca/archives/p1/2004/2004-04-03/html/notice-avis-eng.html#i1
[115] Gooding, D. and A.J. Bodnarchuk. 1994. Field trials of prewetting salt and sand with MgCl2 and CaCl2 brines: Efficiency and effects. Highway Environment Branch, British Columbia Ministry of Transportation and Highways. 7 pp.
[116] Environment Canada, Envirozine, “Protecting the Environment while Maintaining Road Safety in Winter”, Issue 49, Dec. 2004, 5 pp.
[117] Environment Canada, “Case Study # 6 - Winter Maintenance Innovations Reduce Accidents and Costs - City of Kamloops”, May, 2005, http://www.ec.gc.ca/nopp/roadsalt/cStudies/en/kamloops.cfm
[118] Environment Canada, “Biodiversity Portrait of the St. Lawrence – List of Priority Vascular Plants“, Dec. 2002, http://www.qc.ec.gc.ca/faune/biodiv/en/recherche/regions/recherche_regions.html
[119] Finnish Meteorological Institute ,“Estimation of road salt use based on winter air temperature”, Meteorological Applications, vol. 8, Issue 3, p.333-338
[120] Birgisdóttir, Harpa. Ph.D. Thesis, “Life cycle assessment model for road construction and use of residues from waste incineration”, Jul. 2005
[121] U.S. FWHA,” Manual of Practice for an Effective Anti-icing Program”, Nov. 1996.






we will post your article mlb jerseys shop aeroponics vs hydroponics.I will post for our customers to see your articles on your blog nike jersey
ReplyDeleteThis is very interesting, You are a very skilled blogger.
ReplyDeleteI've joined your rss feed and look forward to seeking more of your wonderful post. Also, I have shared your website in my social networks!
My page - Car Insurance For High Risk Drivers - What's Required
It's really very complex in this full of activity life to listen news on TV, thus I only use world wide web for that purpose, and take the latest information.
ReplyDeleteVisit my web blog Esurance Ratings
I think this is among the most important information for me.
ReplyDeleteAnd i am glad reading your article. But want to remark on
some general things, The site style is wonderful, the
articles is really nice : D. Good job, cheers
Feel free to visit my page ; Strom Gas Anbieter
My famіly members every time say that Ι am waѕtіng
ReplyDeletemy time hеre at web, howеѵer I know I am getting know-how everyday by reading ѕuch faѕtiԁious
articles.
Review my webpage keyless remote programming
It's perfect time to make some plans for the future and it's time to be happy.
ReplyDeleteI have read this post and if I could I want to suggest you few
interesting things or tips. Maybe you could write next articles referring to this article.
I want to read even more things about it!
Follow the gossip on the directory
Feel free to visit my homepage :: Somerset Listings
I simply couldn't depart your site prior to suggesting that I really enjoyed the standard info a person supply in your guests? Is going to be back steadily in order to check up on new posts
ReplyDeleteMy homepage Diamond Titanium ring
This is very motivating;.You are a very expert blogger.
ReplyDeleteI've connected your roses feed and look onward to looking for more of your magnificent post.I like the post and I have shared my site air conditioner Montréal.
I like the post and I have shared my site air conditioner Montréal.
ReplyDeleteI have study in Canada and i am working in a Hotel part time. I am studying and work also in Canada. So I like use full time mini split air conditioner.
ReplyDeletewall mounted AC.
mini split AC.
Great post! You have really shared valuable information regarding this topic. And I am looking forward to read more of your articles. But I just wonder if this road salt that contains inorganic chloride salt is considered toxic, then what is the best alternative for salt?
ReplyDeleteEastCoastSalt.com
Iཿve been exploring for a little for any high quality articles or weblog posts in this kind of area .
ReplyDeleteExploring in Yahoo I at last stumbled upon this web site.
Studying this information So iཿm satisfied to exhibit that
I've a very good uncanny feeling I found out just what I needed.
I most certainly will make sure to do not overlook this web site and
provides it a look regularly.
Also visit my web-site: book of ra download